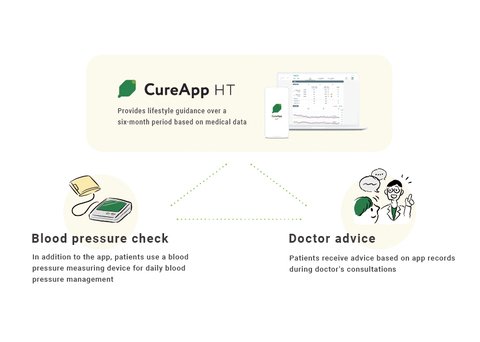
Support lifestyle changes to treat hypertension
TOKYO, November 2, 2022–(BUSINESS WIRE)–CureApp, Inc. (Headquarters: Chuo-ku, Tokyo; CEO: Kohta Satake) announces the start of insurance coverage for its complementary high blood pressure treatment app CureApp HT (hereafter CureApp HT), a DTx solution*1 for the treatment of hypertension in Japan from September 1, 2022. In the future, the app will be prescribed and dispensed to patients with hypertension as a treatment covered by Medicare in healthcare facilities.
This press release contains multimedia elements. See the full press release here: https://www.businesswire.com/news/home/20221031005332/fr/
Assisting lifestyle changes as a treatment for hypertension (Graphic: Business Wire)
After confirming the efficacy and safety of CureApp HT through clinical trials, the results of a phase III Japanese nationwide randomized controlled multicenter trial have been published in EHJ, one of the peer-reviewed journals most respected in the world in 2021. In April 2022, CureApp HT was the world’s first digital hypertension therapy app to receive government approval and the app has been supported by insurance since April 2022. September 1. It is the first time in the world that a DTx treatment solution in hypertension is covered by insurance. Subsequently, CureApp will continue its efforts to market this medical innovation from Japan. The consequences of hypertension are known beyond Japan’s borders, and efforts to correct patients’ lifestyles are a recurring theme around the world. Therefore, CureApp also plans to proactively expand the reach of CureApp HT worldwide, starting with the United States.
Importance of lifestyle changes, current challenges and potential in CureAppHT
The 2019 Guidelines for the Management of Hypertension describe that “lifestyle changes are important not only because they can have a hypotensive effect, but also because they prevent hypertension”. The rules also state that “lifestyle counseling should be given to all patients with hypertension, as it may increase the hypotensive effect and reduce the dosage of antihypertensive medications.” Despite this recommendation, this type of behavioral treatment has had difficulty taking off due to the difficulty of continually enforcing lifestyle changes on patients and the limited duration of consultations with the physician. It is also said that a certain period of guided intervention*2 is necessary for lifestyle changes to take root.
CureApp HT helps connect doctors and patients and promotes lifestyle changes that were previously difficult to maintain, while ensuring maintenance of changed behavior in patients.
*1 Abbreviation for Digital Therapeutics. Products approved for use as medical devices.
*2 Kamioka H, Nakamura Y, Yazaki T, Uebaba K, Mutoh Y, Okada S, Takahashi M. Comprehensive health education combining hot spa bath and lifestyle education in middle-aged and elderly women: one-year follow-up of three- and six-year randomized controlled trial months of interventions. J Epidemiol. 2006 Jan;16(1):35-44. doi: 10.2188/jea.16.35. PMID: 16369107; PMC ID: PMC7560544.
Summary of clinical trials with this product
https://doi.org/10.1093/eurheartj/ehab559
About CureApp, Inc.
CureApp, Inc. is a medical technology company that conducts research and development aimed at creating software for medical devices that use advanced software technology and medical documentation to treat disease. Its purpose is to manufacture and distribute them. The company is dedicated to the development of therapeutic apps, that is, apps used to treat diseases, to become the first in Japan to offer a new health service centered on “achieving a therapeutic effect to treat diseases using of an app”. In August 2020, CureApp was the first company in Japan to obtain medical device regulatory approval for a disease treatment app, in this case a nicotine addiction treatment app, which subsequently obtained medical device approval. approval for reimbursement from the health insurance in December of the same year.
Companies in which CureApp is involved
|
Nicotine addiction |
Regulatory approval as a medical device in August 2020; reimbursement from health insurance and start of prescriptions from December 2020 |
|
Hypertension |
Authority approval as a medical device in April 2022 |
|
NASH (non-alcoholic steatohepatitis) |
In joint development with Sawai Group Holdings Co. / Ongoing clinical trials with Tokyo University Hospital |
|
Alcohol abuse |
App under development with the national hospital organization Kurihama Medical and Addiction Center / Clinical trials underway at Okayama City General Medical Center, Okayama City Hospital |
|
Oncology |
Treatment app for breast cancer patients currently under development with DAIICHI SANKYO COMPANY, LIMITED. |
|
Chronic heart failure |
App under development with our partners at YUMINO Medical Corporation |
In addition, we offer mobile health programs for private companies through our “ascure Smoking Cessation Programme” and our “ascure Specific Medical Support Program for Smoking Cessation”, which utilize the knowledge acquired in the development of these digital therapies for healthcare institutions. These programs have been implemented in more than 230 companies and health insurance companies. In the future, we will successively implement this “Japan-developed digital health solution” around the world, based on the model established in Japan.
CureApp, Inc Company Profile
|
Manager |
Kohta Satake |
|
The main office |
Kodenma-Cho YS Building 4th Floor 12-5, Nihonbashi Kodenma-Cho, Chuo-ku, Tokyo, Japan, 103-0001 |
|
American branch |
CureApp North America, Inc. |
|
Activities |
Development of SaMD (Software as a Medical Device) and mobile health-related services |
|
URLs |
https://cureapp.co.jp/en/ |
The text of the press release, derived from a translation, should in no way be considered official. The only authentic version of the press release is that of the press release in the original language. The translation will always have to be compared with the source text, which will set a precedent.
See the source version at businesswire.com: https://www.businesswire.com/news/home/20221031005332/en/
contacts
[ Renseignements ]
PR Manager, CureApp, Inc.
pr-team@cureapp.jp (Mishima)